Sowing into dry soil profiles in many parts of the southern region may present challenges for growers when it comes to achieving adequate nodulation and nitrogen fixation from legumes.
However, a Grains Research and Development Corporation (GRDC) and South Australian Grain Industry Trust (SAGIT) investment looking at the optimisation of legume inoculation for dry sowing has found that increasing inoculation rates may be the key in achieving adequate nodulation and therefore better nitrogen fixation in such scenarios.
According to the GRDC publication Inoculating Legumes: A Practical Guide, nitrogen fixed by the soil bacteria rhizobia symbiotically with Australia’s pulse legumes has a large national benefit with more than $200 million dollars of nitrogen fixed by pulses annually.
However, legume nodulation is sometimes sub-optimal, because of one or more factors, including stressful sowing conditions.
With more area across the southern region being sown to pulses, paddocks with little or no history of pulse production are likely to benefit from rhizobial inoculation. Factors such as soil acidity, dry sowing and pesticide seed treatments can stress rhizobia and impair inoculant performance.
Dry sowing and inoculants
In 2018, many areas across the southern region received some rainfall in early May with little follow-up rainfall until June, meaning those crops that emerged early were under moisture stress for several weeks. This, in turn, compromised the survival of rhizobia applied in the form of commercial inoculants prior to sowing.
Research conducted by Primary Industries and Regions SA through its research division the South Australian Research and Development Institute (SARDI) has shown that increasing the rate of inoculant applied as peat slurry to seed improves nodulation where soil conditions at sowing are suboptimal.
At Wanilla on the lower Eyre Peninsula in 2017, faba beans were sown into a dry acidic and sandy soil with seed in the ground for four weeks until a germinating rain occurred.
The trials at Wanilla examined the impact of inoculation rate on nodulation, including a half rate, full rate and double rate of peat inoculant.
This research revealed that applying the commercial strain at double the recommended rate resulted in good nodulation, even under the stressful conditions. Similar results were measured in lupin and chickpea trials, where doubling the rate of peat inoculant also increased nodulation in a dry soil.
In 2018, chickpeas treated with moist peat and peat granule inoculants were sown into a sandy soil site at Lameroo, South Australia, which remained dry for 18 days after sowing.
SARDI senior research scientist Ross Ballard says nodulation increased from 2.5 to 5.6 to 8.3 nodules per plant with each doubling of inoculation rate with peat applied on seed (figure 1).
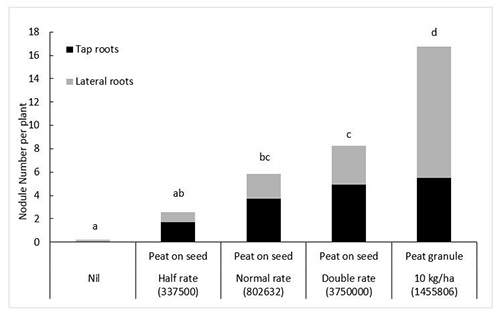
“For this experiment, the peat granules were produced at SARDI to help understand if the application of rhizobia in furrow is as effective as seed application and to improve our understanding of the potential of granulated inoculants,” Mr Ballard said.
“The experimental peat granule produced nearly seven times the number of nodules as was produced by the lowest rate of peat on the seed.
“Most of the increase was in lateral root nodulation, probably the result of the rhizobia being more widely distributed in the soil. The result demonstrates the potential of granules which contain high numbers of rhizobia to improve nodulation.
“The performance of two commercial granules in the trial was comparable to the experimental granule. However, in other trials the number of rhizobia in commercially produced granules has varied and almost certainly affected the consistency of their performance.
“It points to the need for improved quality control, similar to that mandated for moist peat inoculants.”
Inoculants and acid soils
The GRDC publication Legumes in Acidic Soils – Maximising Production Potential in South Eastern Australia states pulse crops (except lupin) and their associated rhizobia are sensitive to low pH.
“The key to achieving consistent and profitable productivity from legumes growing in acidic soils is effective nodulation and seedling vigour,” it states.
Mr Ballard has been researching the acid tolerance of rhizobia strain WSM-1455 (Group F inoculant), which is used in the production of commercial inoculants for faba beans, lentils and field peas.
“Recent assessments of nodulation by WSM-1455 in field trials illustrates the impact that decreasing soil pH has on the number of nodules per plant formed by this inoculant strain,” he said.
“Pulse nodulation decreased rapidly below pH 6 and was negligible at pH 4. The significance of the relationship across a range of growing conditions and legume species demonstrates the key role acidity plays in limiting the nodulation of this legume group.
“It has been suggested that the opportunity to improve the performance of the commercial inoculant strain produced for faba bean and lentil was between pH 4.5 and 5.
“However, based on data from these experiments, it appears this opportunity may extend further to pH 5.5, where decreased nodulation by WSM-1455 is evident.
“Below pH 4.5, nodulation will likely be severely compromised, regardless of the rhizobial strain used and soils must be limed to achieve satisfactory levels of nodulation.”
Researchers are looking to identify rhizobia strains with improved acidity tolerance and have been field testing strains since 2015.
Mr Ballard says overall, the performance of new rhizobia strains across a number of measures has been encouraging and there are good prospects for commercialisation.
“That said, it is expected that the benefits of the new strains will be limited to below pH 4.5. This was borne out at two Victorian sites in 2018 (Stawell, pH 4.2 and Telangatuk, pH 4.1) where faba bean nodulation, even with the new rhizobia, was limited to less than 10 nodules per plant, which is well below the industry benchmark,” he said.
“Improved rhizobia should be seen as an accompaniment, not a replacement for liming. Liming remains important to correct acidification and is critical to the longer-term sustainability of the farming system.
“Even where the improved rhizobia are used, nodulation will be suboptimal below pH 4.5 and liming remains the most effective strategy to improve nodulation. Plant root growth will also likely benefit from the addition of lime and improve overall performance of the pulse crop.”
Pesticide compatibility
Mr Ballard says growers should take care when applying inoculant to seed which has been treated with insecticide or fungicide, especially when sown in dry or acidic soil.
“Where pesticide application to seed is necessary, granular rhizobial inoculant may provide a better option, reducing direct exposure of the rhizobia to the pesticide,” he said.
“Trials at Minnipa, SA, last year by the University of Adelaide looked at the nodulation of field pea when seed had been treated with P-Pickel T (thiram and thiabendazole) compared to untreated seed. Rhizobia inoculants were applied to both the P-Pickel T treated and untreated seed which was sown into an inoculation responsive, dry sandy soil.
“Rhizobial seed inoculation was only adequate in the absence of P-Pickel T, because the fungicide greatly reduced the number of rhizobia on the seed. Inoculation strategies that separate the inoculant from the pesticide will be examined in 2019. In the meantime, granular inoculants provide the best option.”
Assessing nodulation
The GRDC’s Inoculating Legumes: The Back Pocket Guide provides some tips on assessing nodulation of legumes post-sowing. These include:
- Take a few plants from each of several locations in the paddock 8-10 weeks after sowing, to cover paddock variability
- Carefully dig up plants with root systems intact and gently wash roots (e.g. in a bucket of water) to remove soil
- Cut nodules open: pink-coloured tissue indicates active N fixation.
Desired numbers of nodules are given in the sections of the guide for individual legume species.








